THE POWER OF 7-DAY DOSING:
IDELVION OFFERS CONSISTENTLY HIGH
FACTOR IX TROUGH LEVELS IN CHILDREN, ADOLESCENTS, AND ADULTS
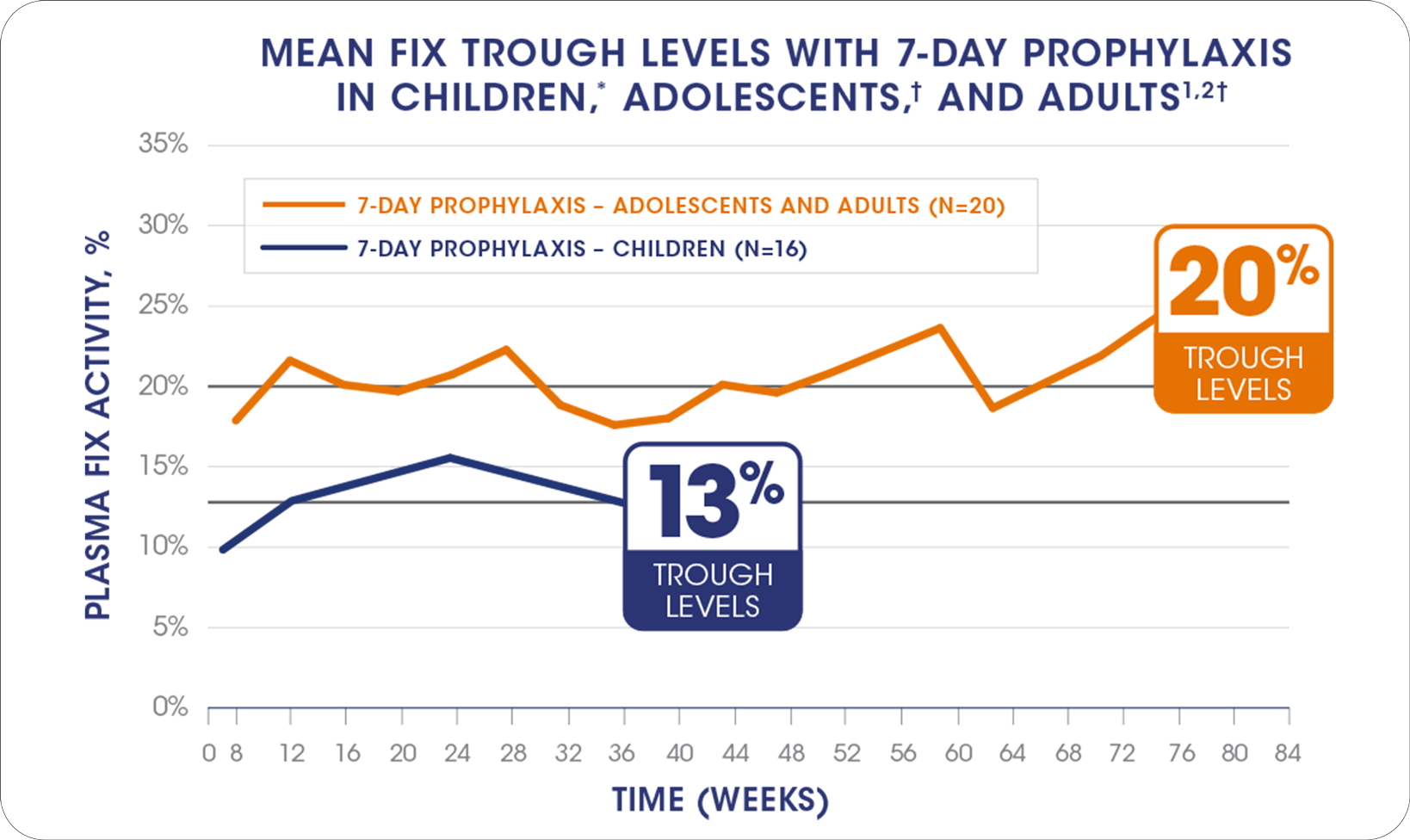
*Only time points with ≥3 observations are plotted. Steady-state mean trough is calculated from all observations at any time point. The mean dose for patients receiving prophylaxis for every 7 days was 47 IU/kg.
†Only time points with ≥3 observations are plotted. Steady-state mean trough is calculated from all observations at any time point. The mean dose for patients receiving prophylaxis for every 7 days was 37 IU/kg.

In pediatric patients <12 years
EXTENDED HALF-LIFE.
EXTENDED TIME
BETWEEN DOSES.
In the pivotal trial, IDELVION delivered a prolonged half-life
- In clinical trials, previous Factor IX products used by subjects had a half-life of 18.6 hours. Previous FIX products refers to plasma-derived FIX or rFIX3
- Half-life is based on a single dose of 50 IU/kg in children (≤12 years)

In adult patients
LONGEST
SINGLE-DOSE
HALF-LIFE OF
ANY FIX.
Half-life is based on a
single dose of 50 IU/kg
in adults ≥18 years
a single dose Of 75 iu/kg PROVIDED factor ix levels above 5% for 14 days
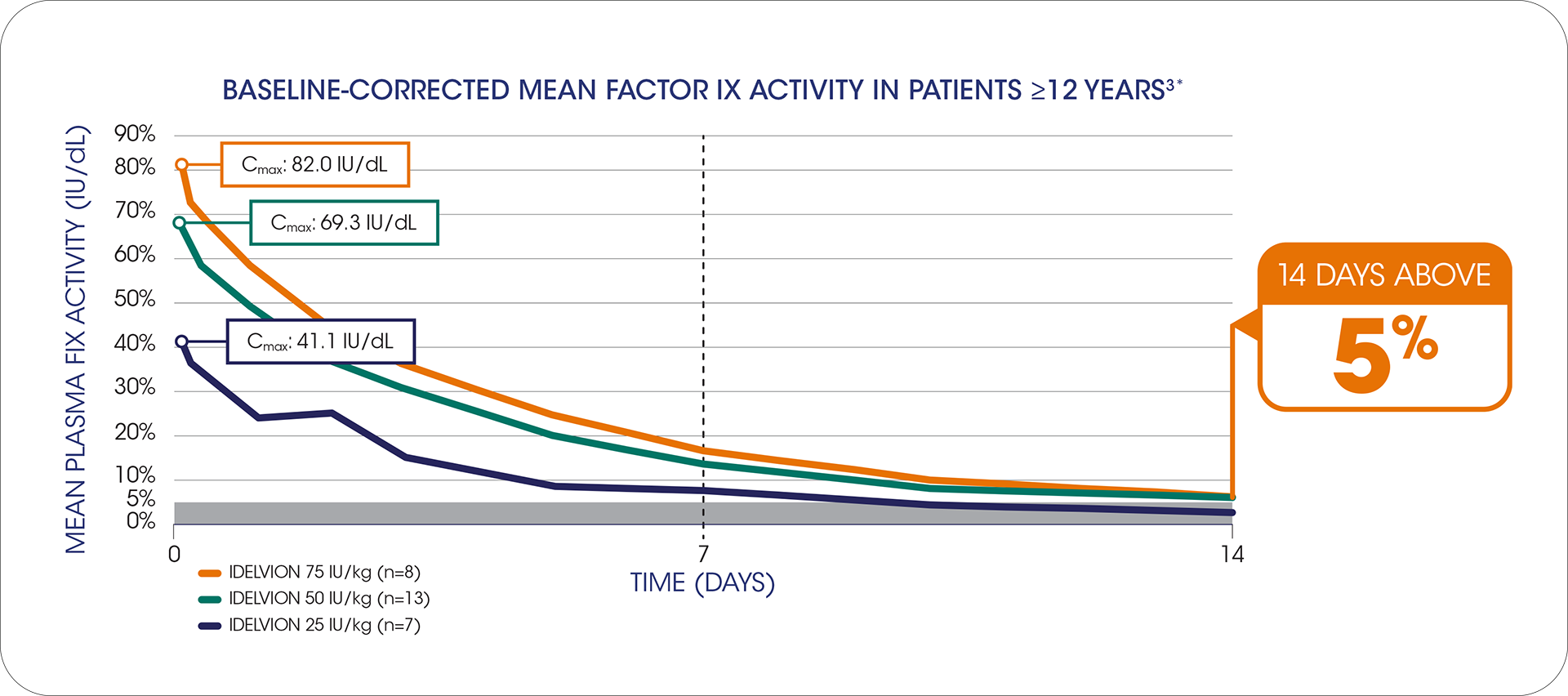
*Data from the phase 1 trial, a first-in-human prospective, multicenter, open-label dose-escalation study to evaluate the safety and PK of 25, 50, and 75 IU/kg rIX-FP in subjects with hemophilia B. Twenty-five subjects were enrolled in order to ensure at least 13 evaluable subjects in the 50 IU/kg dosing group and at least 4 evaluable subjects in both the 25- and 75-IU/kg rIX-FP dosing groups. All subjects received rIX-FP in a nonbleeding state and after a washout period of at least 4 days from their last dose of the previous FIX product.3

Patients who started and stayed on prophylaxis prove IDELVION has powerful efficacy with both 7- and 14-day dosing†
VIEW EFFICACY DATA
IDELVION uses albumin fusion technology to extend half‑life with minimal risk of an immune response
SEE THE IDELVION MOA†Of the 23 subjects in Arm 2, 19 were transitioned from on-demand to 7-day prophylaxis. The median AsBR during prophylaxis treatment was 0.7 (range: 0 to 4.2). Data for Arms 1 and 2 based on matched-pairs design.
References: 1. Gill JC, Roberts J, Li Y, Castaman G. Sustained high trough factor IX activity levels with continued use of rIX-FP in adult and paediatric patients with haemophilia B. Haemophilia. 2019. doi:10.1111/hae.13735. 2. Data on file. Available from CSL Behring as DOF IDL-003. 3. Kenet G, Chambost H, Male C, et al. Long-term safety and efficacy of recombinant coagulation factor IX albumin fusion protein (rIX-FP) in previously treated pediatric patients with hemophilia B: results from a phase 3b extension study. Thromb Haemost. 2020;120(4):599-606 doi:10.1055/s-0040-1705116. 4. Santagostino E, Negrier C, Klamroth R, et al. Safety and pharmacokinetics of a novel recombinant fusion protein linking coagulation factor IX with albumin (rlX-FP) in hemophilia B patients. Blood. 2012;120(12):2405-2411.
