IDELVION BLEED PROTECTION OFFERS CONFIDENCE IN BOTH 7- AND 14-DAY DOSING REGIMENS
PHASE 3 PIVOTAL TRIALS: ADOLESCENTS AND ADULTS ON 7- AND 14-DAY PROPHYLAXIS*
Data from patients who started and stayed on prophylaxis prove IDELVION has powerful efficacy with both 7- and 14-day dosing*


Abbreviations: AjBR, annualized joint bleeding rate; AsBR, annualized spontaneous bleeding rate; IQR, interquartile range.
†IQR is the middle 50% of people in a clinical study.
*Of the 23 subjects in Arm 2, 19 were transitioned from on-demand to 7-day prophylaxis. The median AsBR during prophylaxis treatment was 0.7 (range: 0 to 4.2). Data for Arms 1 and 2 based on matched-pairs design.
Phase 2/3 study design: The safety and efficacy of IDELVION were evaluated in a prospective, open-label, multicenter clinical study of 63 male PTPs with hemophilia B (≤2% endogenous FIX activity) who received at least one infusion of IDELVION. Subjects were aged 12 to 61 years; including 7 adolescent subjects aged 12 to 17. Subjects were treated for up to 27 months.
EXTENSION STUDY: ADOLESCENTS AND ADULTS ON 7- AND 14-DAY PROPHYLAXIS*
The confidence in bleed protection with 7- and 14-day dosing comes from long-lasting efficacy over 4 years2


Phase 3 extension study design: This multicenter, open-label phase III extension study investigated the long-term safety and efficacy of IDELVION for routine prophylaxis and on-demand treatment of bleeds. Hemophilia B PTPs (FIX ≤2%) (n=59) who participated in a phase III pivotal study or who underwent surgery with IDELVION and continued with prophylaxis were enrolled in the study. Enrolled subjects were males aged 13–63 (mean age: 36.1 years). Five subjects were between 12–17 years of age. During the study, subjects were treated for a median (range) of 36.8 (7, 49) months.
For information on pediatric patient data, call CSL Behring Medical Information at 1-800-504-5434 or email MedInfoNA@cslbehring.com.
IDELVION resolved a majority of bleeds with a single on-demand infusion
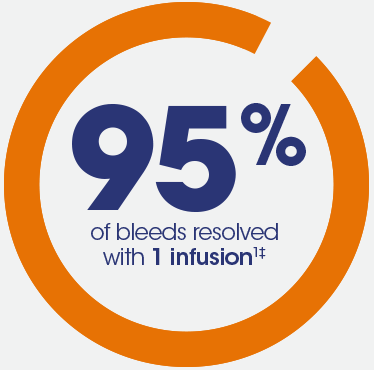

‡In patients not receiving prophylaxis.
IDELVION demonstrated powerful hemostasis in surgery
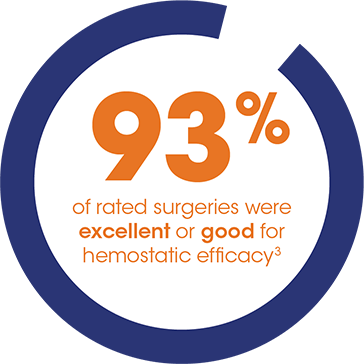

PROLONG-9FP surgical substudy design: This study enrolled male patients, ≤65 years old with severe hemophilia B (FIX activity ≤2%) requiring non-emergency surgery. A total of 30 surgeries have been conducted in 21 patients, including four surgeries in four pediatric patients.

High and sustained Factor IX levels in clinical trials in adolescents and adults
LEARN MORE ABOUT STEADY-STATE FACTOR IX LEVELS§Once well‑controlled (1 month without spontaneous bleeding or requiring dose adjustments on a weekly dose of ≤40 IU/kg), people 12 years and older can be transitioned to 14-day dosing.
§Once well-controlled (1 month without spontaneous bleeding or requiring dose adjustments on a weekly dose of ≤40 IU/kg), people 12 years and older can be transitioned to 14-day dosing.
References: 1. Santagostino E, Martinowitz U, Lissitchkov T, et al. Long-acting recombinant coagulation factor IX albumin fusion protein (rIX-FP) in hemophilia B: results of a phase 3 trial. Blood. 2016;127(14):1761-1769. 2. Mancuso ME, Pan-Petesch B, Lissitchkov T, et al. Long-term safety and efficacy of rIX-FP prophylaxis with extended dosing intervals up to 21 days in adults/adolescents with hemophilia B. J Thromb Haemost. In press. 3. Curtin J, Santagostino E, Karim FA, Li Y, Seifert W, Négrier C. Simplifying surgery in haemophilia B: Low factor IX consumption and infrequent infusions in surgical procedures with rIX-FP. Thromb Res. 2020;188:85-89. 4. Gill JC, Roberts J, Li Y, Castaman G. Sustained high trough factor IX activity levels with continued use of rIX-FP in adult and paediatric patients with haemophilia B. Haemophilia. 2019. doi:10.1111/hae.13735.

