In pediatric patients <12 years IDELVION BLEED PROTECTION OFFERS CONFIDENCE IN 7-DAY DOSING
PHASE 3 PIVOTAL TRIALS: CHILDREN ≤12 YEARS ON 7-DAY PROPHYLAXIS
7-day prophylaxis proven in phase 3 pivotal study


Abbreviations: AjBR, annualized joint bleeding rate; AsBR, annualized spontaneous bleeding rate; IQR, interquartile range.
†IQR is the middle 50% of people in a clinical study.
Phase 3 study design: This was a prospective, non-randomised, international, open-label phase 3 study, with all patients assigned to weekly prophylactic treatment. All patients participated in PK evaluation of 50 IU/kg rlX-FP at study entry. The patients were assigned a dose of 35—50 IU/kg rIX-FP for weekly prophylaxis, at the investigator's discretion. The dose could be adjusted higher or lower, based on bleeding phenotype, physical activity level, and clinical outcome, while maintaining a weekly treatment interval. The active treatment period for the evaluation of safety and efficacy was extended up to 18 months to allow patients to receive continuous treatment with rIX-FP until enrollment in the subsequent extension study. Efficacy and safety assessments were performed at study sites approximately every six weeks.1
EXTENSION STUDY: 7-DAY DOSING IN CHILDREN
The confidence in bleed protection with 7-day dosing comes from long-lasting efficacy evaluated for up to 4 years4

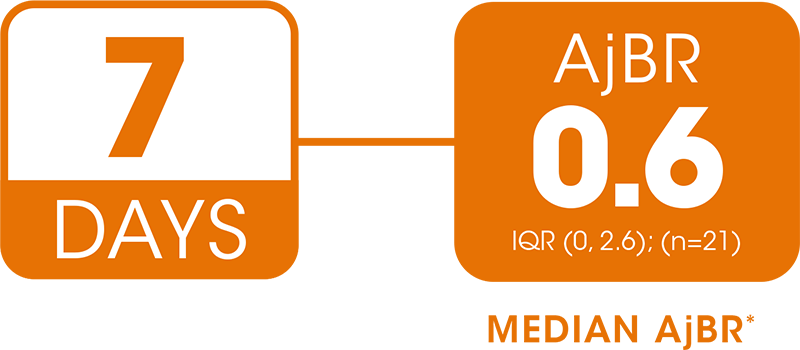
Phase 3 extension study design: Previously treated patients aged < 12 years with moderate to severe hemophilia B (FIX activity ≤2%) enrolled in a 3-year extension study following a phase 3 pivotal study in which they received weekly rIX-FP prophylaxis. Enrolled subjects were males aged 2—11 (median age 7 years). During the study, subjects were treated for a mean (standard deviation) of 37.1 (9.8) months.2
For information on pediatric patient data, call CSL Behring Medical Information at 1-800-504-5434 or email MedInfoNA@cslbehring.com.
References: 1. Santagostino E, Martinowitz U, Lissitchkov T, et al. Long-acting recombinant coagulation factor IX albumin fusion protein (rIX-FP) in hemophilia B: results of a phase 3 trial. Blood. 2016;127(14):1761-1769. 2. Mancuso ME, Pan-Petesch B, Lissitchkov T, et al. Long-term safety and efficacy of rIX-FP prophylaxis with extended dosing intervals up to 21 days in adults/adolescents with hemophilia B. J Thromb Haemost. In press. 3. Kenet G, Chambost H, Male C, et al. Long-acting recombinant fusion protein linking coagulation factor IX with albumin (rIX-FP) in children: results of a phase 3 trial. Thromb Haemost. 2016;116(4):659-668. 4. Kenet G, Chambost H, Male C, et al. Long-term safety and efficacy of rIX-FP in previously treated pediatric patients with hemophilia B: results from a phase 3b extension study. Thromb Haemost. 2020;120(4):599-606.
