
IDELVION Connectsm patient support program

Get IDELVION news that matters to you

Call 1-800-676-4266
Monday–Friday,
8 am to 8 pm ET

Use this guide to start the conversation with your healthcare provider to see if IDELVION is right for you

Recommended for mobile devices
IDELVION offers powerful bleed protection for all ages
TAKE FIX LEVELS TO ANOTHER LEVEL
In adolescents and adults, high steady-state trough levels* regardless of dosing schedule



Get the elevated, sustained factor levels that meet your needs, IDELVION delivered
high and sustained Factor IX trough levels with prophylactic use.
* Steady state is consistent and uniform amount of factor in the body from continued use.
† The average dose for people receiving prophylaxis every 7 days was 37 IU/kg and every 14 days was 73 IU/kg.
‡Half-life is based on single dose of 50 IU/kg in adults (≥ 18years)
High trough levels and a long half-life means
Factor IX stays in your body longer.
What are trough levels?
Trough levels are when your FIX levels are at their lowest before your next injection. Since bleeds are more likely to happen when FIX levels are low, the higher your trough level, the better protected you are from bleeds.

What is half-life?
Half-life is the amount of time for levels to decrease by half after infusion. The longer the half-life, the longer Factor IX stays in your body.

ADOLESCENTS AND ADULTS ON 7-DAY AND 14-DAY PROPHYLAXIS*
In adolescents and adults in pivotal trials, there was a median of 0 spontaneous and 0 joint bleeds*
regardless of whether they chose 7- or 14-day dosing.†
That means the powerful bleed protection of
IDELVION sticks around while you plan your next
adventure, so you can live life the way you choose.


* The median AsBR for people who started and stayed on 7- or 14-day prophylaxis was 0. For people who switched to
prophylaxis from on-demand, the median AsBR was 0.7. AsBR=annualized spontaneous bleed rate.
† Once well-controlled (1 month without spontaneous bleeding or requiring dose adjustments on a weekly dose of
≤40 IU/kg), people 12 years and older can be transitioned to 14-day dosing.
CHILDREN DESERVE POWERFUL PROTECTION
AS THEIR CAREGIVER, YOU CAN PROVIDE IT WITH IDELVION
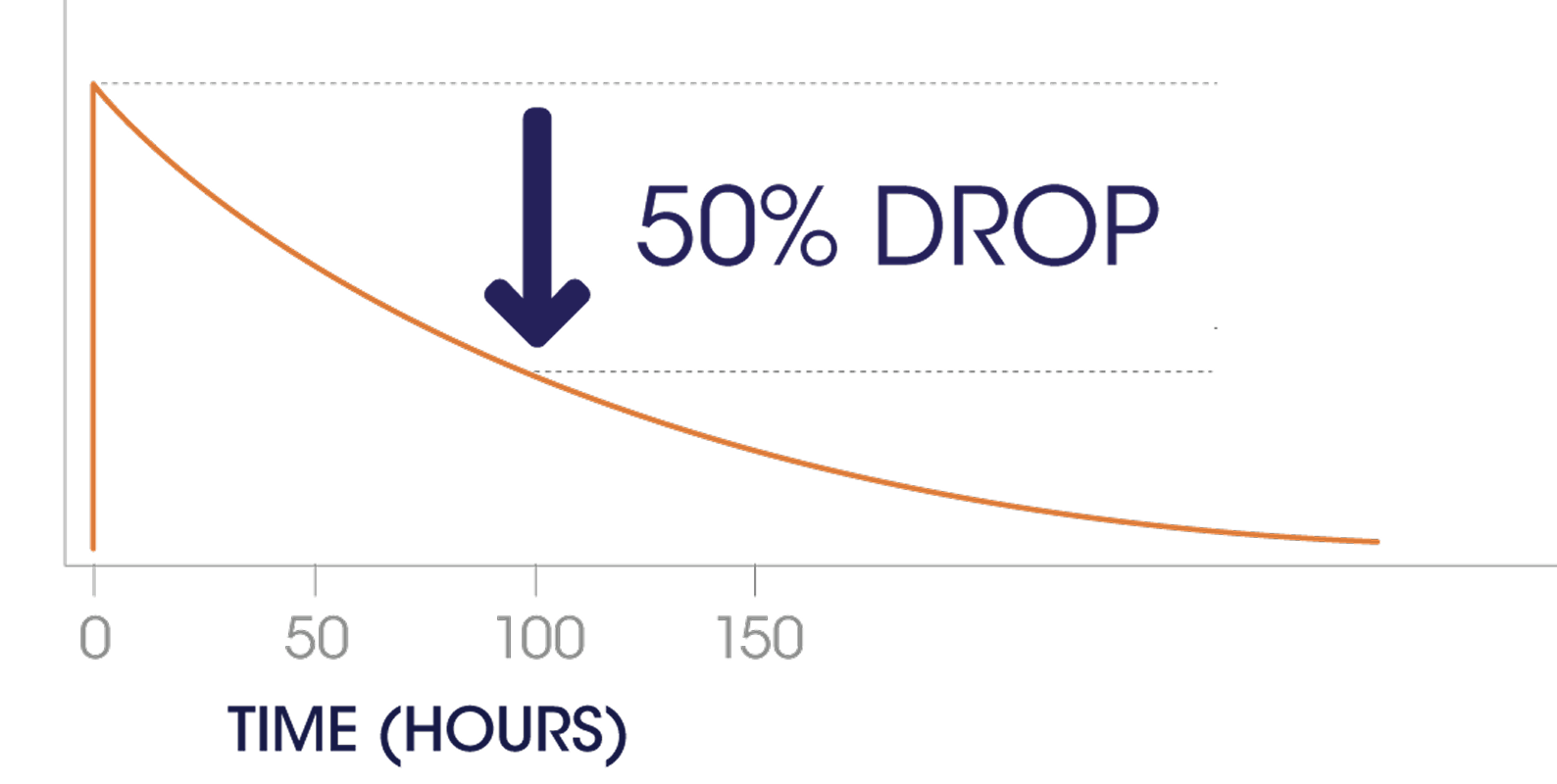
With the longest single-dose half-life of any Factor IX therapy at 90–93 hours,*
IDELVION keeps Factor IX levels high enough to extend protection.


* Based on a single dose of IDELVION at 50 IU/kg in children (< 12 years).
† The average dose for children receiving prophylaxis every 7 days was 47 IU/kg.
High trough levels and a long half-life means
Factor IX stays in your body longer.
What are trough levels?
Trough levels are when your FIX levels are at their lowest before your next injection. Since bleeds are more likely to happen when FIX levels are low, the higher your trough level, the better protected you are from bleeds.

What is half-life?
Half-life is the amount of time for levels to decrease by half after infusion. The longer the half-life, the longer Factor IX stays in your body.
CONFIDENCE IN YOUR CHILD'S BLEED PROTECTION — SUPPORT IN YOUR CARE ROUTINE
In the IDELVION pediatric pivotal trial, children had a median of 0 spontaneous bleeds
per year and less than one joint bleed per year when dosed every 7 days.


† The median annualized spontaneous bleeding rate was 0 on 7-day dosing.
‡ The median annualized joint bleeding rate was 0.99 on 7-day dosing.



